
NIDEK Excimer Lasik Laser EC 5000 CX-111
( Installed at Sri Venkateshwara Nethralaya, RR nagar, Bangalore)

The NIDEK Advanced Vision Excimer Laser (NAVEX) system consists of the OPD-Scan aberrometer and topographer, Final Fit interface software, and the EC-5000 CX excimer laser employing scanning slit and spot ablation capabilities to deliver treatment onto the cornea.
The OPD-Scan (Optical Path Difference Scanning System) is a combination aberrometer and topographer that uses the principle of spatial dynamic skiascopy to measure the aberrations of the eye and placido disk topography to measure the corneal shape. The unit uses over 8000 data points to plot the aberrations of the eye and the corneal topography. In addition to aberrometry and topography, the OPD-Scan provides autorefractometry, keratometry, and pupillometry functions integrated into one unit, allowing accurate data registration between the different types of examinations. Owing to this integration of functions, the OPD-Scan is currently employed for surgical planning and preoperative and postoperative evaluations for a variety of refractive and intraocular procedures.
The aberrometry measuring method consists of an infrared light-emitting diode housed within a chopper wheel with slit apertures. The receiving system consists of a photodetector array that converts the time differences of stimulation into dioptric power maps, which correlate the refractive error in a two-dimensional map at the entrance pupil. The dioptric power maps or refractive maps are displayed as “OPD maps” from which traditional Zernike-based maps out to the eighth order can be derived. In contrast to traditional Hartmann-Shack and Tscherning type aberrometers, the OPD exploits time differences of stimulation rather than positional differences of data points to map the aberrations of the eye. This difference of time principle, which has been well established for many years in autorefractometry, allows the measurement of highly aberrated eyes and eyes with large refractive gradients (Fig. 1). The OPD-Scan has a comparatively wide measuring range of −20 to +22 D of sphere and up to 12 D of cylinder. An added benefit is that aberrometry and topography are performed on the same instrument; therefore, the axis of alignment is the same with accurate registration of data.
 |
|
Fig. 1. Example of OPD-Scan measurement of an eye with Sunset Syndrome with a 36 Diopter refractive gradient across the open pupil and a 3 mm OPD refraction of +10.52 − 12.45 × 69. |
Corneal topography is measured by employing a highly sensitive placido ring method. An arc step method is used to calculate the slope of the corneal surface at each ring edge and elevation.
Clinically, the OPD-Scan provides standard corneal topography and wavefront information. The wavefront data plots total wavefront maps, higher-order maps, and Zernike graphs cataloging the aberration profile along with the associated root mean square (RMS) values. The point-spread function (PSF) allows simulation of the optical effects of the measured aberrations. The PSF can be simulated for the total wavefront profile, the higher-order profile, and the effects of the individual types of aberrations such as coma or trefoil. This information can be useful in the subjective confirmation of patient symptomatology and as a patient teaching tool to simulate the effects of the aberrations and explain them in layman terms. Additionally, the Strehl ratio value is provided, which generates a global index of the PSF that quantifies the optical quality of the eye.
Unique to the OPD-Scan are refractive diopter maps termed internal OPD and OPD maps. The OPD map presents the spatially resolved refractive and aberrometric status of the entire optical path of the eye. The OPD internal map displays the refractive status of the eye owing to internal aberrations by subtracting the effects of the corneal front surface from the total aberrometry. This map allows one to determine whether the source of the aberrations is corneal, internal, or a combination. The source can be a critical factor in the clinical outcome of refractive surgery. For intraocular surgery, the internal OPD map allows determination of the centration of the intraocular lens (IOL) and the optical effect of the surgery. Additionally, corneal or lenticular astigmatism can be determined and quantified.
For refractive surgery, various indices allow determination of the corneal vertex, the pupil center, and the photopic and mesopic pupil centers. Preoperatively, this information is vital to define the center of the treatment; postoperatively, these indices can be used to determine the centration of the procedure.
The Final Fit software uses aberrometry, topography, or both to develop the ablation algorithms. This interface software allows modification and simulation of a host of parameters, including optical and transition zones, target refraction, the effective optical zone, and the treatment based on only corneal or wavefront data.
The EC CX III uses scanning slit and spot ablation to deliver the wavefront ablation onto the cornea. The beam is Gaussian with a spot size of 1 mm. The optical zone can be varied from 3 to 6.50 mm, and the transition zone can be varied out to 10 mm. NAVEX allows the treatment of primary refractive surgery candidates and patients who have had suboptimal outcomes from previous refractive surgery. Performing the refractive correction with the slit ablation and the wavefront correction by multipoint ablation allows up to six spots to be delivered simultaneously to reduce treatment times. A cyclotorsion module allows compensation for cyclotorsion based on iris details that may occur from the sitting (OPD) to supine positions (EC-5000 CX III). Digital images of the iris pattern are compared between the two positions to maintain centration and detect cyclotorsion. Centration during surgery is maintained with a 60- or 200-Hz eyetracker that tracks the physiologic pupil during the treatment.
In addition to conventional ablations, three treatment modalities are available using NAVEX. All three modalities employ an aspherical treatment zone to maintain a large effective optical zone. The surgeon can use full wavefront correction, termed OPD-CATz (OPD-guided custom aspherical transition zones), which uses topography and aberrometry to determine the ablation algorithms. Another option is CATz (customized aspherical transition zones), which uses solely topography data to determine the treatment based on the corneal wavefront. The surgeon also has the option to perform treatments without wavefront ablations yet maintaining a suitable effective optical zone based on an optimized aspherical transition zone (OATz) treatment. OATz forms the base treatment for OPD-CATz and CATz.
Various excimer laser manufacturers have reported large increases in spherical aberration after excimer ablation. This higher-order aberration has been implicated in a variety of night vision disturbances, along with a generalized decreased in best-corrected visual acuity (BCVA).
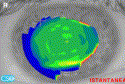
NAVEX uses an ablation algorithm that specifically addresses the issue of spherical aberration to treat corneal aberrations. Essentially, this method involves an increase in the diameter of the transition zone and a reduction in the optical zone, creating an optically seamless integration of the optical and transition zones and nascent cornea. Preliminary results reported by Paolo Vinciguerra, MD, and Arturo Chayet, MD, on human eyes confirm that there is a reduction in the amount of induced spherical aberration [1]. The advantages of this unique treatment method are that it actually decreases ablation volume, creates a prolate cornea, and increases the effective optical zone. By moving what Vinciguerra refers to as the “red ring” on corneal topography past the pupillary excursion diameter and reducing the severity of contour change, the spherical aberration that would normally affect the PSF is effectively reduced. The red ring on instantaneous (or tangential) topography often has a deep red ring after excimer laser ablation. The severity of the color signifies abrupt dioptric power changes in the cornea between the optical zone, transition zone, and nascent cornea. This abrupt change induces the type of spherical aberration that can cause significant adverse patient symptomatology postoperatively. The diameter of the ring signifies the effective optical zone Fig. 2, Fig. 3.
 |
|
Fig. 2. Small effective optical zone after a conventional ablation for −5 D signified by the red ring on instantaneous topography. (Courtesy of Paolo Vinciguerra, MD.)
|
 |
|
Fig. 3. Large effective optical zone after a NAVEX customized aspherical transition zone treatment for −6 to 1.5 D × 90 signified by the lack of a red ring on instantaneous topography. (Courtesy of Paolo Vinciguerra, MD.) |
By introducing patented ablation algorithms in the treatment zones, the NAVEX platform reduces the abrupt dioptric power changes and, consequently, the spherical aberration induced by many conventional excimer lasers. The prolate nature of the cornea has the added advantage that the coupling of aberrations between the lens and cornea is not disrupted to the same extent as in conventional ablations, which induce oblate corneas. Use of the OPD-Scan and Final Fit software allows simulation of the postoperative aberrometry and topography maps. Customization of the effective optical zone to a specific pupil size is also possible within the Final Fit software.
Initial results from several investigative sites have produced encouraging outcomes, such as maintenance or increases in contrast sensitivity and the subjective quality of vision. The advent of this unique ablation algorithm allows refractive surgeons to taper treatments that maintain quality of vision in photopic and scotopic conditions.
NAVEX has been used for the treatment of primary eyes and the retreatment of patients who have had suboptimal outcomes from previous refractive surgery (NAVEX enhancements).
A total of 132 primary eyes with myopia and astigmatism were treated using the CATz modality by Arturo Chayet, MD, (Mexico) and Mihai Pop, MD, (Canada) (personal comunication, October, 2002). Preoperative sphere ranged up to −8.25 D and cylinder up to −3 D. These subjects comprised a representative sample from refractive surgery centers, and the patients were not preselected for a certain level of RMS error or visual acuity preoperatively before wavefront correction. The range of refractive error was not limited such that it would skew toward positive results. All treatments were targeted for emmetropia. Ninety-eight percent of the patients were within 1 D of the intended correction (Fig. 4). Eighty-seven percent of the patients were within ± 0.50 D of the intended correction. Uncorrected visual acuity (UCVA) at 1 to 3 months postoperatively is shown in Fig. 5. Ninety-three percent of the patients achieved 20/20 vision or better, with 45% achieving 20/15 or better UCVA. The CATz approach was safe, with 95% of the eyes maintaining or gaining lines of BCVA (Fig. 6A).
 |
|
Fig. 4. Accuracy for 132 eyes treated with CATz. |
 |
|
Fig. 5. Uncorrected visual acuity post CATz treatment. |
|
|||
|
Fig. 6. (A) Safety post CATz treatment. (B) Change in RMS for 6 mm of CATz and conventional ablation patients. (C) Change in contrast sensitivity in CATz-treated eyes 3 months postoperatively (n = 26). |
|||
The RMS induced by CATz was compared with that induced by conventional ablations (Fig. 6B). The average RMS decreased for higher-order aberrations, trefoil, coma, and spherical aberration (Fig. 6B). Twenty-six patients underwent contrast sensitivity testing preoperatively and postoperatively (Fig. 6C). Eighty-four percent of the patients maintained or gained contrast sensitivity 3 months postoperatively, showing relatively quick rehabilitation of visual quality.
Subjectively, patients expressed a high degree of satisfaction with NAVEX treatments. These results support the safety and accuracy of the treatment of myopia and astigmatism by NAVEX. Postoperatively, patients have excellent unaided visual acuity.
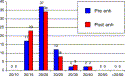
NAVEX enhancement was performed on 63 patients who had suboptimal outcomes from previous refractive surgery [2]. Seventy-one eyes with myopia, hyperopia, or mixed astigmatism were treated owing to clinically significant symptoms that reduced the quality of vision. All of the eyes had previously undergone LASIK or photorefractive keratectomy. The three treatment modalities outlined previously were used depending on the specific requirement for each case. Preoperative sphere ranged from −2.5 to +3.5 D, with cylinder up to 3 D. The average RMS preoperatively was 0.883 μm. The preoperative compared with postoperative BCVA is shown in Fig. 7. Postoperatively, the average RMS was reduced to 0.878 μm. Seventy-eight percent of treatments had a reduced RMS post NAVEX enhancement.
 |
|
Fig. 7. NAVEX enhancement. Preoperative versus postoperative BCVA. |
In addition to its use in the NAVEX system, the OPD-Scan is a useful clinical tool in everyday clinical practice. By detecting and quantifying optical aberrations and defining whether the source of the aberrations is from the front surface of the corneal tear film or other internal structures, the clinical efficacy of fitting a contact lens to correct the aberration can be determined before spending valuable time and money on the typical empirical trial methods traditionally employed. The etiology of nonoptimal post-fitting outcomes can be diagnosed accurately and corrected. The OPD-Scan is also useful in differentiating whether a patient's reduction in vision is caused by optical abnormalities or other disorders of the retina or optic tract. As more is learned about this dynamic field, diverse clinical applications will be implemented to use the technology to the fullest clinical benefit.
Common Eye Problems | Diabetic Eye Disease | Retinal Detachment | Glaucoma | Eye Camp | Opticals | Medical Ethic | Diabetic Diet