Carl Zeiss Meditec Platform with MEL-80 Excimer Laser- Courtesy , , , , , , , e
The eye can be thought of as a sophisticated optical instrument that automatically adapts to its environment. It has automatic systems for adjusting the light level (pupil/iris) and focus (accommodative lens). The eye can also have a range of common optical errors that limit its performance. The dominant errors in most eyes are myopia (nearsightedness), hyperopia (farsightedness), and astigmatism (asymmetrical focal power). These errors have been corrected for centuries by adding a lens in front of the cornea (glasses or contact lenses). Modern laser refractive surgery has been driven by efforts to modify the structure of the cornea itself.
Traditionally, the degree of these errors in the eye has been measured by a trial-and-error process. A trial corrective lens is placed in front of the eye, and patient feedback gives information about whether the vision is better or worse. The phoropter, trial lens kit, and to some extent the autorefractor are the primary instruments used to gather this refractive error information. The subjective refraction (or manifest refraction) method is the primary means of determining the proper patient corrective lens prescription and has, for the past 15 years or so, formed the basis for calculating ablation profiles to be applied to the cornea. The errors in the eye's optical system, however, are not always limited to focus and astigmatic errors. For example, it is possible for the focal power to change at different locations across the pupil. These effects cannot be described purely in terms of focus or astigmatism, because they relate to changes in focus and astigmatism as a function of position. Thus they are called “higher-order” aberrations, while focus and astigmatic error are defined as “low-order” aberrations. Examples of higher-order aberrations are changes in the focal power as a function of pupil diameter (predominantly spherical aberration), increased power in the upper half of the pupil, decreased power in the lower half (vertical coma), and increased or decreased power left to right (horizontal coma). These effects can be small and have little effect, or they can dominate visual quality, causing starburst, glare, image ghosting, and even monocular diplopia. Until recently, it was only possible to measure the higher-order aberrations of the eye in controlled laboratory conditions, in vivo. Thanks to the advent of modern aberrometers, it is now possible not only to measure these higher-order aberrations routinely in a doctor's office but to enable physicians to base corneal refractive surgery on this information.
Definition of wavefront error
The wavefront of the light that is transmitted through an optical system is an imaginary surface that remains normal to the direction of propagation at all cross-sectional points within the optical pathway. For a perfect eye, focused at infinity, the wavefront of the light collected by the optics of the eye would be part of an aspheric surface, which would converge on the back of the eye to create a (diffraction-limited) spot on the retina. Because of the reciprocal behavior of light (that is, it traverses the same path in either direction), it is possible to measure this wavefront from light that is scattered or reflected from the retina (after it has been projected onto it). This is the primary measurement principle of the Carl Zeiss Meditec WASCA (Wavefront Supported Customized Ablation) device (Jena, Germany), produced jointly with Wavefront Sciences (Albuquerque, New Mexico), which markets the same device as the Complete Ophthalmic Analysis System (COAS) in the United States. In operation, the WASCA emits a small beam of light, projecting this onto the retina. The light scatters from the retinal surface (fovea), is collected by the lens and cornea, and is then “projected” out of the eye. A perfect, emmetropic eye would completely collimate this light. The resulting wavefront would be a perfect flat plane wavefront, perpendicular to the direction of propagation. Any deviations from a perfect plane wave are the result of optical errors in the optic system (ie, the eye) and are called the wavefront error of the system.
Custom ablation: definition
We define custom ablation as an ablation profile designed to meet optical correction requirements for a specific individual eye. The recent introduction of diagnostic aberrometry into refractive surgical practice has put higher-order optics into the standard vocabulary of refractive surgeons. This development has also raised new issues concerning the design of ablation profiles. For example, aberrometry has now made it possible for us to study which aberrations are being induced by current treatment profiles [1]. Accordingly, in our view, wavefront-guided ablation comprises an important component but not the total sum of custom ablation. In addition, we must consider a number of independent variables contributing to a scheme designed to give us control over the modification of corneal shape. These include the factors affecting the accuracy of laser energy delivery to the cornea and ablation biophysics, as well as epithelial and biomechanical responses within the cornea. This article describes the current and near-future components of the Carl Zeiss Meditec system, designed to achieve true custom ablation within the cornea.
Aberrometry: WASCA
The WASCA aberrometer (Fig. 1) is based on the Shack-Hartmann wavefront sensing principle. Briefly, it operates thus: a lenslet array collects incident light emerging from the eye. Each lenslet then creates a focal projection onto a CCD camera array. The position of the spots on the CCD array relative to a reference location (as would be produced by a flat wavefront) is used to determine the actual wavefront slope of the incident light onto a particular lenslet. The combination of this array of slopes in a topographic manner leads to the calculation and digital reconstruction of the incident wavefront. This wavefront can be either displayed directly from the raw data by “zonal reconstruction” (as a result of the very high resolution of the WASCA) or broken down into a collection of shapes of varying amplitude (eg, the Zernike expansion series). The authors will now delineate some of the specific design features of the WASCA that provide for increased accuracy and reproducibility of wavefront measurement in practice.
 |
|
Fig. 1. WASCA aberrometer unit, showing narrow head design that enables fellow eye to “see past” the device, to minimize instrument myopia and enable fellow-eye targets to study dynamic accommodation. (Courtesy of Wasca, Zeiss, Meditec.) |
Lenslet array resolution and spot quality
Lenslet resolution has a direct impact on the accuracy of wavefront detection, the dynamic range within a wavefront to be detected, and the reproducibility of detection. Resolution not only affects the concordance between the measured and actual wavefront but also influences whether a wavefront measurement can even be obtained from a particular aberrated eye.
The lenslet array incorporated in the WASCA is based on the core patented technology of Wavefront Sciences (Albuquerque, New Mexico) and is comprised of the most compact (highest-resolution) lenslet array available worldwide. It is constructed using methods similar to those for creating modern integrated circuits. That is, the lenslets are designed using a computer-generated mask, which is transferred to the surface of a fused-silica substrate using photolithography and reactive-ion etching. This process results in a lenslet array with known and extremely accurate characteristics. Fig. 2 shows some examples of the focusing data for a small portion of the incident light. Fig. 3 shows a small portion of the actual lenslet array. The full array consists of 1452 lenslets arranged in a rectangular array of 44 × 33. The shape of each lenslet surface is spherical, but each lenslet is a 144-μm square section of this surface. In this way, there is 100% fill of the focusing array, so that no light can “leak” through between lenslets. This arrangement reduces stray light that could cause interference or background effects that reduce spot localization accuracy. The full array measures 6.5 mm × 4.8 mm. The WASCA lenslet array enables approximately 800 lenslets to collect light from a 7-mm entrance pupil, with an effective lateral resolution of 210 μm. Comparison with other commercially available aberrometers with respect to the number of lenslets collecting from a 7-mm entrance pupil is shown in Table 1.
 |
|
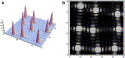
Fig. 2. Small portion of the image from a lenslet array. (A) shows the irradiance distribution incident on the CCD detector, and (B) is the resulting CCD image pixel by pixel. The location of these spots is the key information that is used to determine the wavefront. |
 |
|
Fig. 3. Scanning electron micrograph of a portion of the WASCA lenslet array. Each lenslet is in fact square and comprises a depression of approximately 1.6 μm with a diameter of 144 μm. Note the 100% fill (ie, no gap) between lenslets. |
|
Table 1.
Comparison of commercially available aberrometers with respect to the number of lenslets (or equivalent) collecting light within a 7-mm entrance pupil |
|
|
System name (manufacturer) | Type | Spots in 7-mm pupila | Lenslet pitch (resolution)/μm |
|
|---|---|---|---|---|---|
|
|
WASCA/COAS (Carl Zeiss Meditec) | Shack-Hartmann | 800 | 210 |
|
|
|
Wavescan (VISX) | Shack-Hartmann | 222 | 400 |
|
|
|
LADARwave Custom Cornea (Autonomous) | Shack-Hartmann | 172 | 450 |
|
|
|
Wavefront Analyser (Wavelight) | Tscherning | 98 | 600 |
|
|
|
Zywave (Bausch & Lomb) | Shack-Hartmann | 74 | 700 |
|
|
|
KR-9000 (TOPCON) | Shack-Hartmann | 74 | 700 |
|
|
|
VFA (Tracey) | Retinal Ray-Tracing | 80 | variable |
|
|
a
Calculated From the lenslet pitch and obtained from published sources. |
The other most common method of creating lenslet arrays is by producing molded monolithic lenslet modules. These are made by an embossing process in plastic. Some of the weaknesses of this method are that the lenslet shape is less predictable and that it cannot produce a 100% fill, thus potentially enabling stray light to “leak” between lenslets.
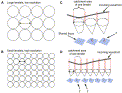
To understand why lenslet size affects resolution and how this in turn affects the accuracy of wavefront detection, the authors present the illustration in Fig. 4. Essentially, the smaller the lenslets, the more of them can fit into the catchment area of the pupillary zone, and hence the higher the resolution. Smaller lenslets have smaller diameters and therefore collect a smaller sample of the incoming wavefront. The smaller the sampled area from the incoming wavefront, the less “averaging” of the wavefront slope within one lenslet occurs, and thus the more focused the spot projected onto the CCD detector is. The more focused the spot is on the CCD detector, the more likely it is that (1) it can be detected with good positional accuracy, and (2) it is a true representation of the slope of the incoming wavefront into that lenslet.
 |
|
Fig. 4. (A,B) The smaller the diameter of the lenslet, the more can be contained in an array and the greater the number of lenslets collecting light from the entrance pupil. (C) shows a portion of wavefront containing a nonlinear slope entering the catchment of the leftmost lenslet, but effectively a linear slope entering the middle and rightmost lenslets. The sample of the wavefront in the case of the leftmost lenslet has a nonlinear structure; therefore, the lenslet will scatter light over a large region, causing a diffuse and irregular focal spot. In the case of the middle and rightmost lenslets, light will be properly focused (F), producing a sharp peak. (D) Increasing the number of lenslets or decreasing the diameter of the lenslets allows more frequent sampling. Now each lenslet is picking up effectively linear slopes from the same wavefront as in (C). Because of the smaller sampling area, the nonplanar portion of the incoming wavefront is now divided into quasi-planar segments to produce well-focused projections (F) onto the CCD array. |
Fig. 5 demonstrates the CCD capture output obtained from a mild keratoconic eye, using either a 400-μm or 200-μm resolution lenslet array. This example clearly demonstrates how lenslet size and resolution affect the quality of the data acquired and hence the reliability of the wavefront calculated.
 |
|
Fig. 5. Shack-Hartmann lenslet array CCD images from an eye with mild keratoconus using either a 400-μm or a 200-μm resolution array. The same central area of each image is sampled and zoomed ×3 to demonstrate the difference in spot quality afforded by increased resolution. In this example, the lower resolution array can produce spot projections that would lead to inaccurate centroid determination, thus affecting the accuracy of the calculated wavefront. |
Spot quality and wavefront detection accuracy
If the position of these focal spots cannot be determined accurately, the entire accuracy of the device will suffer. The focal spot is sampled by a fixed number of pixels on the CCD camera, digitized and stored (see Fig. 2). The digital image is then broken up into a number of areas of interest (AOIs), encompassing one focal spot in each AOI. These AOIs are processed to determine the position of the spot using an algorithm. The thresholded centroid algorithm used has been shown to have the accuracy of a fraction of a pixel when the spot covers several pixels [2], [3].
In order for the centroid to be determined accurately, the light incident on the detector must form a distinct grid of spots. To this end, as demonstrated above, the lenslet must sample a portion of the light that has low enough aberrations (essentially, a wavefront of constant slope) to form an ideal detectable focal spot. If, however, the incident light on a particular lenslet has a complex wavefront, the focal spot's peak intensity is reduced, and the spot will be significantly blurred and spread out (see Fig. 4). In this case, the centroid algorithm may not be accurate, or it may not be able to determine a spot position at all. An algorithm that is optimized to measure the location of a distinct spot accurately will perform poorly on blurry spots. Conversely, an algorithm that is optimized for detecting centroids in blurry spots will not be optimal for measuring distinct spots. The best overall system, then, will be one that optically makes each spot distinct. This goal is achieved by using as many lenslets as possible over a given wavefront, so that the wavefront is as close to being planar as possible over each lenslet.
Spot crossing
In Shack-Hartmann sensing, the range of motion of the focal spot on the detector is correlated to the dynamic range. Thus, if the focal length of the lenslets is large compared with the diameter of the lenslets, focal spots can actually cross over when the presenting wavefront possesses a large change in the gradient from one lenslet to the next. The WASCA has been specifically designed with a very fine-pitch lenslet array and an extremely short focal length to match, to provide a large dynamic range (6 diopters [D]). A patented system forces a lenslet-focused spot to “drop out” before it can cross over to the detector field of the adjacent lenslet. Hence spot-crossing is impossible with the WASCA aberrometer.
These features, the large dynamic range and the elimination of spot crossing, explain why the WASCA is robust in its ability to provide a detectable, reproducible wavefront measurement even in highly aberrated eyes.
Zonal reconstruction
The wavefront analysis process consists of three main steps: detecting the centroids of each focal spot, comparing the positions to a reference to produce a slope map, and finally, reconstructing the wavefront.
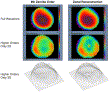
The reconstruction of the wavefront may be displayed as a point-by-point integration of the two-dimensional raw slope map—a zonal reconstruction (Fig. 6). Or the wavefront may be reconstructed according to a defined mathematical fit (eg, to the Zernike polynomial series)—a modal reconstruction. If the zonal resolution is low, the zonal reconstruction method will yield highly pixelated data. If, however, the zonal resolution is high (as with the WASCA), this raw pixel density is sufficient to provide graphic two-dimensional representation of the wavefront without resorting to artificial smoothing of the raw data. The advantage of viewing a zonal reconstruction representation is in the lack of smoothing produced by the “binning” of raw data into a defined polynomial series (particularly if one does only lower analyzing, ie, up to the sixth or eighth Zernike order). Modal reconstruction has certain advantages in that each polynomial possesses specific optical connotations and hence can provide quantization of clinical data. However, zonal reconstruction has the advantage of producing much higher spatial frequency and therefore less smoothing (Fig. 7). Thus, zonal reconstruction may provide a better starting point for calculating customized ablation profiles in highly aberrated eyes.
 |
|
Fig. 6. Drawing showing a wavefront being converted by the Shack-Hartmann sensor into a two-dimensional grid of individual wavefront slopes (done as a function of spot displacement on the CCD image). These elementary wavefront slopes can be “lined up” to produce a reconstruction of the original wavefront. If the resolution is high (ie, consisting of a large number of elementary wavefront samples), the reconstruction is smooth and of good fidelity. |
 |
|
Fig. 7. Full wavefront and higher-order two-dimensional and three-dimensional displays for a single wavefront measurement acquired from an eye with a visually significant “central island” post-photorefractive keratectomy (PRK). The plots in the left column are based on a Zernike expansion representation of the wavefront up to the eighth order, while those in the right column are based on direct zonal reconstruction from the raw slope data. Detection of the optical irregularity produced by the central island is smoothed out by description of the wavefront data with Zernike polynomials, while zonal reconstruction with high spatial resolution (equivalent to a Zernike reconstruction to the 24th order) enables detection of the optical irregularity. (Courtesy of Gunter Grabner, MD, Salzburg, Austria.) |
Seidel method for predicting manifest refraction
It is understood that higher-order aberrations can be partially compensated in the visual system by the use of sphere and cylinder lenses. That is why some eyes become more myopic at night when the pupil becomes larger: increasing pupil size increases the amount of spherical aberration in the eye, an effect which in turn can be partially compensated by increasing the power of a spherical lens placed in front of the eye. The influence of similar, higher-order astigmatic terms, such as Z(4,2) and Z(4,−2), will have an effect on the total cylindrical power that a patient calls for in manifest refraction. In brief, the Seidel method provides a means for calculating the manifest equivalent spherocylindrical refraction based on combining the lower-order Zernike terms for sphere and cylinder (ie, sphere, Z[2,0]; cylinder, Z[2,−1] and Z[2,−1]) with the relative influence of higher-order (third and above) polynomials.
The use of the Seidel method effectively makes the WASCA into an accurate “autorefractor.” (See clinical data below).
Specific design features that improve performance
To optimize the quality of the light focused on the retina, the WASCA is designed to maintain full wavefront sensor resolution and accuracy at all magnitudes of hyperopia and myopia (+6 D to −15 D) through a patented precorrection of the light “injected” into the eye.
The multiple display options provided are summarized in Box 1.
Summary of the display options available from the WASCA aberrometer
Wavefront map
Power map
Displays for data interpretation
Dynamic analysis features built in
Open data format for export
The WASCA is negligibly affected by vibration, as it acquires a complete data set within 13 milliseconds.
The eye's optical system is not static and is constantly shifting or focusing. Using the rapid acquisition time and proprietary high-speed analytical systems, the WASCA is capable of obtaining multiple full wavefront measurements per second. Software enables the user to acquire wavefronts at 15 Hz over a period of up to 30 seconds. The WASCA is designed to be able to acquire a total of 450 independent wavefront measurements within a period of 30 seconds. These may be viewed as a continuous movie clip, fostering an appreciation for the stability and reproducibility of the ocular wavefront. The clip provides a dynamic analysis of the wavefront and facilitates determination of the true average prescription, which may not be just the average of a few measurements. Algorithms will soon be released that make possible automatic multiple wavefront acquisition, instantaneous statistical analysis, and the delivery of the “best” wavefront for the purposes of planning customized ablation (Daniel R. Neal, PhD, personal communication, 2003).
This rapid-sequence acquisition also enables dynamic aberrometry to be performed. Vogelsang, Panagopoulou, and Pallikaris [4] have studied the dynamic changes in higher-order aberrations occurring during the accommodation process. Dynamic study of aberrations will become increasingly important, particularly as the use of aberrometry may aid in understanding optical performance for specific tasks (eg, optimizing distance vision at night versus optimizing near and midrange vision indoors). Complex superposition of particular aberrational states of the eye may allow us to induce asymptomatic multifocality (eg, for the treatment of presbyopia). Realistic classification of such intermediate states can only be performed by true dynamic studies, because averaging between static measurements is unlikely to provide adequate answers. Fig. 8 shows an example of how dynamic higher-order aberrations can be, even in the resting state of the eye.
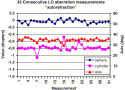 |
|
Fig. 8. Thirty-two consecutive “autorefraction” measurements made by WASCA, showing sphere (blue), cylinder (pink), and axis (red) for each consecutive independent measurement after head repositioning. Major deviations coincide with blinks. The standard deviations for these measurements are sphere, 0.049 D; cylinder, 0.089 D; axis, 1.57°. |
Accuracy and reproducibility
Lower orders: WASCA as an autorefractor
Salmon et al [5] formally studied the accuracy and reproducibility of the COAS (WASCA) in young myopic patients with respect to predicting sphere and cylinder obtained by autorefractor and manifest refraction. For lower-order aberrations, in Seidel mode, WASCA was compared with manifest refraction, dry and cycloplegic. The reproducibility of multiple measurements of the same eye under cycloplegia was tested by 30 repeated measurements, including head repositioning. A single case example of reproducibility is shown in Fig. 8. Including blinks, the standard deviation of the sphere term was < 0.05 D, with the cylinder < 0.09 D. A summary of the study results is shown in Table 2. The repeatability coefficient for cycloplegic data acquisition was 0.15 D [6]. These studies show that WASCA is at least as accurate as subjective refraction (the standard of comparison), but more reproducible.
|
Table 2.
WASCA/COAS accuracy and reproducibility |
|
|
Mean error/D | Standard deviation/D |
|
|
|---|---|---|---|---|
|
|
Dry manifest | −0.140−20×011 | 0.32 |
|
|
|
Cycloplegic | −0.060−18×001 | 0.26 |
|
|
Accuracy of “autorefraction” by WASCA compared with both dry and cycloplegic manifest refraction. The mean reproducibility for multiple measurements of the same eye is also shown. |
Higher orders: WASCA reproducibility for higher-order measurements in vivo
The higher-order terms, as measured by the Zernike polynomials, showed similar reproducibility to the lower-order measurements. Fig. 9, Fig. 10 shows the standard deviation (in μm) of each Zernike coefficient for higher-order aberrations measured for a single case. The repeatability of in vivo measurements was better than 0.05 μm. Due to the high accuracy and rapid data acquisition rate the WASCA aberrometer can overcome instantaneous variability in wavefront Zernike coefficients that may occur due to tear film or accomodative instability, and provides the ability to determine median values for the whole wavefront over a period of time.
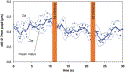
|
|
Fig. 9. Time-based (x-axis) measurement of Z(4,0) variation in a nonaccommodating eye. Wavefront measurements were acquired at a rate of 7 Hz for a period of 30 seconds with a patient looking at a distance target with the fellow-eye. All instantaneous values of Z(4,0) are shown (blue dots), surrounding a running average (solid blue line), and the 95% confidence limits of variation (two standard deviations) (dashed blue line). It can be appreciated how a single instantaneous measurement of Z(4,0) does not necessarily provide a reliable measure of the overall mean value and may be significantly different from the optimal value to be used for planning customized ablation. |

|
|
Fig. 10. Zernike polynomial coefficient reproducibility (standard deviation) for a sequence of 30 measurements of right and left eyes after head repositioning. The x-axis shows the Zernike ordinal number. Reproducibility is shown in microns. |
Surgeon-controlled custom ablation: the Carl Zeiss Meditec CRS-Master
Overview
Surgeon Control
To date, for the most part, laser manufacturers have designed ablation profiles by starting with a theoretical, spherocylindrical-based ablation profile (cf Barraquer [7]; Munnerlyn [8]), which was adjusted through experimental iteration (“nomogram adjustment”) to achieve the desired effect on ocular defocus. For the past decade of excimer laser corneal refractive surgery, the surgeon's control of the ablation profile has been limited. Most manufacturers have allowed the surgeon control only of the intended diameter of the fully corrected optical zone or of the size of the “transition” zone. The function of the CRS-Master (Carl Zeiss Meditec AG, Jena, Germany) is to offer the surgeon further variables to consider and control—including wavefront, topography, and tissue safety-limits.
Using this software application, surgeons integrate patient clinical examination data with corneal surface-shape information and wavefront (WASCA) data, giving themselves control over the ablation profile for each individual eye.
The first CRS-Master release took place outside the United States in September 2003. This section provides an overview of the current version features together with features that will be included in future versions. Graduated clinical studies documenting efficacy and safety will be used to determine the release sequence of particular features.
Safety
Barraquer [9] taught in his 1980 textbook on keratomileusis that to prevent progressive keratectasia following keratomileusis, the corneal cap should not be thicker than 300 μm. In the average 550-μm cornea, this would leave a residual stromal thickness (RST) of 250 microns. If the human cornea had an average thickness of 900 μm, any patient would be able to have his or her refractive error, wavefront, and asphericity optimized within these safety limits. In reality, the human cornea has a mean thickness of 515 μm, with 95% of it lying between 440 μm and 590 μm when measured with very high frequency (VHF) digital ultrasound [10]. Therefore, based on the individual optical errors and corneal thickness constraints of each particular patient, certain compromises will need to be made on the degree of prolateness, or the flattening of the wavefront. Compromises in surgery require clinical judgment. Clinical judgment is the function of surgeons. Therefore, the CRS-Master was developed to integrate a sophisticated, theoretical optical application with clinical judgment, effectively allowing surgeons into the loop of ablation profile design. Given specific desired outcome variables (eg, standard ablation, optimization for night vision, or tissue-saving algorithms [TSA]), preset buttons enable the surgeon instantly to review suggested profiles. The CRS-Master custom mode enables the surgeon to individualize the wavefront data incorporated into the desired ablation profile, and automatically addresses asphericity to optimize the reduction in spherical aberration. Given that increasing the asphericity for a given correction increases ablation depth, future versions will allow the surgeon to control the asphericity factor manually. Thus the surgeon himself is able to design the optimum distinct ablation profile for a specific eye. Ablation profile design is assisted by three-dimensional maps that allow the surgeon to visualize each step of the optimization process.
Data integration
Wavefront data
The CRS-Master wavefront component is based on the WASCA system.
Corneal surface-shape data
The corneal surface data is used to compute, according to beam angular-dependence, the laser energy adjustment required to deliver a constant fluence to the corneal surface (see further discussion). This process ensures that the delivery of excimer laser pulses is optimized for achieving accurate shape changes on the stromal surface according to the angle-of-incidence of the beam to the corneal surface in the periphery.
The extended range, including some of the original Meditec TOSCA (Topography Supported Custom Ablation) functions, will eventually be included in the CRS-Master, incorporating all the commercially available (except in the United States) algorithms already implemented for the MEL70, for the correction of decentrations [11], [12] and small optical zone enlargement [12]. The coordination of corneal anterior surface wavefront information (derived from topography) and the whole-eye wavefront derived from the WASCA is also in development.
Patient clinical data
Pupil size, corneal thickness, and subjective refraction form clinical input components. By integrating clinical constraints into the ablation profile design, the surgeon is able to make informed decisions regarding the patient's particular anatomy and visual requirements. In addition, the CRS-Master allows individualized microkeratome precision data to be entered (mean and standard deviation), and provides the surgeon with a statistical analysis predicting the probability of crossing the 250-μm barrier under the flap for a particular ablation profile set-up.
Background principles: achieving the “perfect” ablation
The concerns surrounding customized ablation of the cornea can be divided into two main issues: (1) optimization of ablation profiles (are we getting the ablation profile we think we are getting?), and (2) customization of ablation profiles (what kind of ablation profile is required by this particular eye?).
Optimization of ablation profiles
Laser fluence control
Projection correction
Standard, theoretically determined, nomogram-adjusted profiles that have been employed to date in corneal refractive surgery have been shown to induce far more higher-order aberration than was predicted. Mrochen et al [13] have reported and calculated the influence of the effective illumination area and possible reflection losses that occur during laser–tissue interaction. When a laser spot is projected onto the curved corneal front surface, moving a scanning-spot from the center of the cornea toward the limbus results in increased effective illumination area and hence less fluence. Reflective losses should also be accounted for to optimize energy delivery to the surface. These effects in turn lead to a decrease in the intended ablation depth per laser pulse (Fig. 11). These phenomena, if not accounted for, result in effectively smaller fully corrected ablation zones and optical distortion of the intended spherocylindrical profile, leading to increases in higher-order aberrations, particularly fourth order (spherical) aberration. Optimization of the ablation profile will be necessary to ensure that lasers are indeed removing the intended tissue volume. To produce this result, laser delivery systems will have to be able to take into account the actual shape of the cornea when calculating an ablation profile. The CRS-Master uses the integrated TOSCA module to calculate the fluence-correction factor required at each location on the cornea and to ensure the accuracy of transferring the desired ablation energy to the cornea.
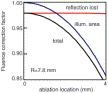
|
|
Fig. 11. Graph showing how projected laser spot fluence drops off as a function of distance from the center (blue line) for a spherical surface of radius 7.8 mm. At 4 mm from the center there is a 15% loss of fluence. Reflection losses are shown (red line) and amount to approximately a 2.5% loss of energy. |
Reflection correction
The stromal surface denuded of epithelium or exposed under a flap is appreciably shiny, because it is reflecting light. Therefore, the excimer laser energy delivered to the cornea is consumed in photoablation; a small but not negligible proportion (approximately 2.5%) is reflected from the surface of the cornea. The angle of the incident laser beam onto the curved corneal surface will be a factor in determining the magnitude of these losses. The reflectivity of the surface also changes over time, particularly during ablation, as a function of evaporation and fluid shock waves within the tissue. The CRS-Master is designed to consider these effects and compensate for them, in optimizing the delivery of spots to achieve the desired stromal curvature change.
Control for tissue responses
Fourth-order spherical aberration and third-order coma are the major higher-order aberrations induced by corneal refractive surgery [14], [15].
Epithelial changes
Using VHF digital ultrasound arc scanning (Artemis: Ultralink, St. Petersburg, Florida) [16], [17], [18] in conjunction with Orbscan II (Bausch & Lomb, Salt Lake City, Utah), Reinstein and colleagues have begun to characterize epithelial and biomechanical responses occurring as a result of laser in situ keratomileusis (LASIK) [19], [20].
For the epithelium, they describe a non-linear and paradoxical power shift in the cornea due to epithelial profile changes. The epithelium is responsible for a myopic power shift as a result of lower myopic ablations, but, paradoxically, it is also responsible for a hyperopic shift in higher myopic ablations. Knowledge of this epithelial behavior and of the magnitude of these changes is helpful in understanding features of ablation-profile adjustments that were hitherto only accounted for by empirical (nomogram or iterative) adjustments. The effect and design of transition zones are also important to the understanding of epithelial profile responses after profiled photoablation.
Biomechanical changes
Biomechanical changes are responsible for thickening of the cornea peripheral to the ablation zone and flap (Fig. 12)[16]. Roberts [21] has proposed a model for this effect and concludes that biomechanical considerations will have to be taken into account for accurate wavefront guided correction of the cornea [22]. This increase in thickness occurs peripherally, but, because of cross-linking of corneal lamellae, it also has an effect centripetally within the cornea. The result is a centrifugal progressive reduction of intended tissue removal, and hence a radially progressive effective loss of intended flattening. This loss leads to an increase in spherical aberration and an effective reduction in the fully corrected optical zone, as described by many investigators [23], [24].
|
|
|
Fig. 12. VHF digital ultrasound-based thickness profiles of the stromal component of the cornea before (2) and 6 months after (5) LASIK for correction of −5.00 D. Difference map (before-minus-after) demonstrates thinning in the center of approximately 70 μm consistent with the refractive ablation within a 6.5-mm zone. Peripheral to the ablation zone (within the 8-mm zone of the cornea), the stroma thickened after surgery by approximately 20–25 μm. (From Reinstein DZ, Silverman RH, Raevsky T, Simoni GJ, Lloyd HO, Najafi DJ, et al. A new arc-scanning very high-frequency ultrasound system for 3D pachymetric mapping of corneal epithelium, lamellar flap and residual stromal layer in laser in situ keratomileusis. J Refract Surg 2000;16:423; with permission.] |
Prolate optimization function
Spherical aberrations followed by coma are the major higher-order aberrations that present in normal eyes [25]. However, they are present, for the most part, with an order of magnitude considerably less in amplitude than the aberrations induced by current ablation profiles. Therefore, to reach the point of being able to offer an improvement on the natural aberrational structure of a virgin cornea, we will first have to minimize the induction of aberrations when treating the defocus (spherocylindrical) component.
Power versus shape
Barraquer's method of keratomileusis was based primarily on the central corneal shape, while wavefront derived sculpting is based on measuring and altering power within the entrance pupil. In Barraquer's keratomileusis, myopic refractive error was treated by modification of the radius of curvature of the outer surface of the cornea. From a theoretical standpoint, removing a spherically based ablation profile should not significantly increase spherical aberration. But in keratomileusis, Barraquer [26] did notice and consider that these higher order aberrations were being induced, and published on aspheric (parabolic) keratomileusis. As shown in Fig. 12, degradation of the intended stromal shape change occurs in a centrifugal fashion; there is progressively more thickening of the stromal layer and hence a reduction in the intended peripheral flattening. This explains the observed increase in spherical aberration after myopic ablations, or what is more commonly referred to as a reduction in the effective optical zone [37]. Patel and Marshall [27] in 1993 reported on a model to predict the ideal postoperative shape of the cornea for minimizing spherical aberration. They mathematically predicted that the postoperative corneal contour should conform to a flattening ellipse with a shape factor (p) = 0.65 to 0.85, corresponding to an asphericity factor (Q) of −0.35 to −0.15 or an average Q of −0.25. Flattening ellipses are prolate surfaces, and hence the CRS-Master offers a tool, namely the “prolate optimization function” (POF), to optimize the post-operative asphericity of the cornea, and hence reduce the induction of higher order aberrations.
Compensation for induced spherical aberration
Spherical aberration is induced for the most part as a result of biomechanical shifts (discussed earlier in this article) that effectively produce a radially progressive degradation of the intended ablation profile. This degradation induces an asphericity change that can be counteracted by increasing the ablation depth as a function of radius. This effect is achieved by the use of aspheric profiles—a technique suggested by Seiler [28], who compared them with standard Munnerlyn profiles.
The POF is thus a tool that is designed to produce a variably aspheric ablation profile so as to optimize the postoperative asphericity and minimize postoperative spherical aberration. Because increasing the asphericity of an ablation profile —while maintaining the central radius of curvature constant—requires a deeper ablation centrally, the CRS-Master will provide the option of switching this feature off and returning to a standard Munnerlyn profile. The authors are currently testing a version that will provide the surgeon with a slider that allows control over the degree of prolate optimization for a particular cornea. If the slider is set to 100%, the ablation profile asphericity is maximally optimized. A setting of 0% would denote that the postoperative asphericity factor is the result of a simple conventional spherocylindrical ablation. In conjunction with tissue constraints and individual patient visual requirements, the surgeon will be able to vary the percentage of POF included in the ablation profile to provide a particular eye with its individualized “best option” ablation profile asphericity.
Compensation for the effect of the flap
Surgically induced coma is mainly a function of ablation centration and of flap-induced changes that are due to the incomplete nature of the keratectomy to hinge the flap. This observation raises the question of whether flap-induced aberrations could be characterized and used in ablation profiles. Reinstein and Goes [29] studied two different microkeratomes (Hansatome, Bausch & Lomb, St. Louis, Missouri; M2, Moria, Antony, France) in a prospective, randomized, controlled evaluation. The Hansatome and M2 were found to induce significantly different aberrations. But, although the mean response for flap-induced aberrations could be characterized for each microkeratome, the variances (standard deviation) of these changes from eye to eye were very large. Thus, the predictions that could be based on these data would be ineffective for customizing the treatment of a particular normal eye with initially low amplitudes of higher-order aberrations. Perhaps the newer generation of femptosecond-based keratomes [30] will make possible more predictable flap architecture and hence more predictable mechanical changes within the cornea, making flap-induced aberrations more predictable and easier to account for in ablation profile design.
Customization of ablation profiles
Practical considerations
Compared with photorefractive keratectomy (PRK) of the early and mid-1990s, LASIK has become the patient's procedure of choice, providing clear advantages in terms of patient comfort and rapid visual recovery. Improvements with respect to pain management and the prevention (or treatment) of post-PRK haze have led to a resurgence in the popularity of surface ablation. It appears, however, that in expert hands the safety of the two procedures is comparable [31]. The induction of higher-order aberrations in LASIK by the creation of the flap itself has now been reported by many [32]. It remains to be seen whether surface ablation carries advantages with respect to customized ablation. However, the difference in the degree of simplicity for enhancement surgery may still keep the bias toward LASIK, given that the correction of higher-order aberrations, even by surface ablation, often requires more than one treatment.
Treating inherent higher-order aberrations
Once there has been a reduction (ideally, elimination) of the higher-order aberrations induced by surgery, it is reasonable to consider the correction of innate naturally occurring higher-order aberrations of the eye, which tend to be of relatively low amplitude.
The WASCA system, as discussed earlier in this article, is specifically designed to make possible the acquisition of wavefront data from highly aberrated eyes. It therefore makes sense to include ablation profile characteristics based on higher-order aberrations with high amplitude, such as are encountered in corneas that have undergone previous refractive surgery.
The MEL80 excimer laser
The MEL80 excimer laser was designed from the ground up to meet the requirements of higher-order customized ablation. The MEL80 operates with a distinct Gaussian beam profile by means of a passive stabilization system (patented), with a 0.7-mm effective ablation spot size at a shot frequency of 250 Hz and a proprietary non-random shot-distribution pattern based on thermography studies, to minimize cumulative surface heating. The patented Gaussian beam profile stabilization system ensures a constant beam profile that does not require user calibration. The shot frequency affords a considerably foreshortened ablation time: a −5.00-D 6-mm zone spherical ablation takes only 15 seconds. Myopic treatments may be performed up to a 7-mm fully corrected ablation zone. The corneal ablation range extends to the 10-mm zone to ensure optimization of hyperopic and other blend zones. The infrared active video tracking system with automatic thresholding and pupil center detection samples at 250 Hz, and the physical delay time for the total system is 2 to 3 milliseconds. The MEL80 has a small footprint (3.3 m2 including bed) and was designed to withstand being wheeled repeatedly over a 2-cm bump without the need for servicing or mirror realignment. Many aspects of the laser-delivery system have been optimized to ensure that energy is delivered efficiently from the laser head to the cornea: features include a very short and direct beam-path length, a vacuum (not inert gas) pathway, and the patented cone for controlled atmosphere (CCA), which produces a virtual environmental cone for the path between the laser aperture and the cornea, ensuring consistent air-flow and tissue debris removal.
Validation of the MEL80 for use in the correction of higher-order aberrations
To validate the use of the MEL80 for the correction of higher-order aberrations, Carl Zeiss Meditec performed laboratory closed-loop experiments. The MEL80 was used to create transparent polymer phase plates with a defined profile that would represent a specific Zernike coefficient of determined amplitude on the polymer surface. The resulting ablated phase plate surface shape was verified independently using a profilometer and was then used to aberrate a collimated planar wavefront beam and generate a wavefront distortion to be detected by WASCA. The WASCA and profilometry measurements were used as measures of accuracy (concordance) in the ability to produce a given wavefront aberration on a phase plate. The WASCA measurement was then imported into the CRS-Master workstation to generate an ablation profile designed to reverse the specific aberration induced.
Fig. 13 depicts a profilometer-based topography measurement and horizontal wavefront section measured after a collimated beam has passed through a phase plate ablated to produce a Z(3,−1) Zernike shape. The concordance between the measured horizontal section and the intended Zernike polynomial can be seen to be very high.
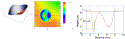
|
|
Fig. 13. Laboratory validation of WASCA and the MEL80 excimer laser as components of a wavefront guided ablation system. Three-dimensional plot of a planned Z(3,−1) Zernike polynomial to be ablated into poly(vinyl acetate) (PMMA) (left), three-dimensional profilometry of the surface of the PMMA plate after ablation (middle), and a horizontal section (red line) showing the actual profile achieved (right). The attempted shape (red curve) is seen to be very close to the achieved shape (blue curve). |
Using the CRS-Master
Box 2 summarizes the parameters that are entered into the CRS-Master for ablation profile planning.
Data the CRS-Master enables the surgeon to import, integrate, and control for ablation profile design
The surgeon imports the wavefront and corneal shape data before being taken to the CRS-Master main control panel (Fig. 14). Here the surgeon enters the manifest refractive error, as well as the target refraction. The treatment refraction is automatically calculated. The procedure is selected (LASIK versus surface ablation), and flap characteristics, with residual stromal thickness safety limits (generally set at 250 μm), are entered via a submenu window. Corneal thickness (central minimum) and scotopic pupil size are entered, while the near-scotopic pupil size obtained from the WASCA analysis (performed in the dark) is displayed for comparison.
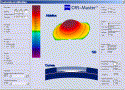
|
|
Fig. 14. Panel for surgeon-controlled custom ablation with a prototype version of the CRS-Master. This window is attained after importing WASCA and TOSCA data. The left-hand column (going from top to bottom) contains fields for entering the manifest refraction, target refraction, type of procedure, and wavefront selection or deselection (with a submenu for user-defined wavefront components). Standard or preset suggested ablation profiles, including tissue-saving algorithms (TSA) and night-vision, can be chosen, or customized surgeon control can be selected to control the POF and treatment zone diameter. The central area of the display contains a user-defined selection of displays, including three-dimensional representation of the ablation profile, wavefront, topography, and others, as well as a two-dimensional cross-sectional representation of the cornea, flap and ablation profile, chosen to depict predicted total ablation depth within the cornea. Shading within this two-dimensional cross-section represents the increased depth that may be produced by the standard deviation of the keratome used (data that is entered by the user in a separate pop-up panel). The right-hand column (going from top to bottom) shows the WASCA-derived pupil size, user-entered scotopic pupil size, patient identification data, and preoperative keratometry and asphericity (from TOSCA) (Q). The pachymetry window allows user entry of the thinnest central thickness, and features calculated fields showing the minimum thickness required for treatment as well as the predicted residual stromal thickness. The predicted postoperative asphericity (Q) is estimated and displayed. These fields update instantly as ablation profiles are modified by the user (eg, modification of POF, selected wavefront components, or treatment diameter). |
A Wavefront control panel enables the surgeon either to deselect WASCA input or to add the higher-order aberrations into the profile. There is also a submenu window that allows the surgeon to select only specific aberrations to be incorporated (eg, only spherical aberration, while excluding coma).
The surgeon now has the option of clicking on ablation profile presets to examine the profiles suggested by the CRS-Master. The “Standard Wavefront” profile preset produces an ablation profile that has a fully corrected optical zone of equal diameter to the scotopic pupil size, with a POF setting of 100% and inclusion of the higher-order aberrations to be corrected. The preset for a “Standard SCA” (standard sphere-cylinder-axis treatment) still incorporates the POF but excludes higher-order aberrations. Another preset, the tissue saving algorithm (“Tissue Saving”) sets the fully corrected optical zone below the analysis pupil size, while maintaining 100% POF. These presets are visually tested by the surgeon's inspection of a cross-sectional corneal profile map that demonstrates the ablation profile with respect to the corneal thickness profile, flap profile, and ablation depth, thus enabling him to determine visually which preset is safely applicable.
The surgeon who is a more advanced user may select the “Customized” preset button. The fully corrected optical zone diameter and (eventually) the POF sliders become active. The surgeon can modify these by studying the cross-sectional corneal profile map to titrate the parameters and their proportion and keep the ablation depth within safety limits. As the sliders are moved, a separate read-out provides the predicted RST value in real time.
A further safety feature provides the ability to determine the 95% confidence limit for the minimum RST that can result, based not simply on the mean thickness of the keratome but on the mean and standard deviation of the microkeratome. We know that the RST limit of 250 μm is set to avoid breaching a much lower RST that would, in fact, produce a destabilized and ectatic cornea. This function, therefore, allows the surgeon to determine the actual probability that the RST will end up at or below 180 μm, as suggested by Reinstein et al [33].
Clinical outcomes of custom ablation using the Carl Zeiss Meditec systems
Wavefront supported custom ablation versus conventional ablation
Photorefractive keratectomy
Professor Dieter Dausch (University of Hanover, Germany) performed a prospective, consecutive, nonrandomized study to compare WASCA (30 eyes) with conventional myopic PRK (47 eyes) using the MEL70 G-scan excimer laser (1.8-mm true gaussian flying spot, active tracker) [34]. Spherical equivalent mean (± standard deviation [SD]) myopia treated was approximately −4.00 D (±2.00 D) with a range extending to −8.00 D in both groups. Outcomes at 1 year were compared. Accuracy analysis revealed that improvements in uncorrected visual acuity (UCVA) continued between the 6-month visit and the 12-month visit. In the WASCA-treated group, the percentage of eyes that were within ±0.5 D of intended correction was 87% at 6 months, reaching 97% at 12 months. In efficacy testing, 100% of WASCA-treated eyes compared with 89% of conventionally treated eyes were 20/20 or better at 1 year. Similarly, 83% of WASCA-treated eyes compared with 68% of conventionally treated eyes were 20/16 or better. In safety comparison, no eyes lost two lines or more in best spectacle corrected visual acuity in either group, but 6% of eyes in the conventional versus 0% of eyes in the WASCA group lost one line. A gain of two or more lines was observed in 53% of the WASCA group versus 6% of the conventional PRK group.
In a noncomparative study, Nagy et al [35] reported on wavefront supported PRK for myopia and myopic astigmatism in 150 eyes. The mean (± SD) preoperative spherical equivalent was −4.02 D (±1.04 D) (range: −1.5-D to −6.5-D spherical correction and 0-D to −2.5-D cylindrical correction). At 6 months, 78.4% of eyes were within ±0.25 D of intended correction, and 98.6% were within ±0.5 D (100% within ±1.0 D). Efficacy showed uncorrected visual acuity of 20/20 or better in 80.7%, with 100% seeing 20/30 or better. No eyes lost lines of best-corrected visual acuity, and 20% gained one line, with 7% gaining two lines. The RMS for higher-order aberrations changed from the preoperative value of 0.32 to 0.42 at 6 months.
These favorable wavefront-guided comparisons appear also to hold for hyperopic PRK (H-PRK). Nagy et al [35] reported on the comparison of WASCA (40 eyes) and conventional H-PRK (40 eyes) in a prospective, consecutive, nonrandomized study using the MEL70 G-scan excimer laser (all treatments performed by a single surgeon). Spherical equivalent mean (± SD) myopia treated was approximately +3.00 D (±0.8 D) in each group. Follow-up was 6 months. While the mean postoperative refraction was similar in the two groups (implying no difference in nomogram discalibration between groups), accuracy analysis showed that the percentage of eyes within ±0.5 D of intended correction was 82.5% and 67.5% for the WASCA and conventional groups, respectively. Efficacy analysis revealed 15% of WASCA-treated eyes compared with none of conventionally treated eyes were 20/15 or better. While there were similar numbers of cases losing one and two lines of best-corrected visual acuity, a gain of one line was observed in 20% of the WASCA group versus only 5% of the conventional H-PRK group.
Laser in situ keratomileusis
Reinstein et al compared the efficacy and safety of MEL70 G-scan LASIK with and without WASCA higher-order aberration data added to the standard myopic treatment profile of consecutive patients presenting at the London Vision Clinic, London, United Kingdom. Two consecutive series of eyes were treated by conventional ablation profile (44 eyes), followed by WASCA wavefront supported custom ablation (36 eyes). All surgery was performed by one surgeon, and all postoperative examinations were performed by a masked observer. Average follow-up was 3.2 months for all eyes. Each group had a comparable starting mean (± SD) and range of spherical equivalent refraction (approximately −4.00 D [±1.60 D]; range −1.25 D to −7.75 D). Efficacy was found to be similar between groups. For conventional versus WASCA-treated groups, UCVA was 20/20 or better in 84% versus 83% respectively, 20/25 or better in 92% versus 91% respectively, and 20/30 or better in 100% in both groups. Six percent (2/36) of eyes in the WASCA group gained two lines of vision versus none in the conventional group, although this difference did not attain statistical significance (Fisher's exact P = 0.23). However there were significant differences noted in safety. While no eye in the wavefront treated group lost one line of best corrected visual acuity, 13.6% (6/44) of the conventional group lost one line (Fisher's exact P= 0.03). (No eyes lost two lines or more in either group.)
It therefore appears that for PRK, improved efficacy as well as safety can be demonstrated by adding the eye's wavefront to the standard ablation profile. On the other hand, LASIK efficacy did not appear to benefit from the inclusion of WASCA data in the MEL70 WASCA study. Improved PRK over LASIK results in wavefront guided treatments could well be due to the shallower overall keratectomy in PRK (no flap), which produces a lesser induction of higher-order aberrations due to biomechanics. In LASIK, flap-induced aberrations may overshadow the low-amplitude, naturally occurring higher-order aberrations. Perhaps preselection of those LASIK candidates with initially high amplitudes of higher-order aberrations would make it possible to demonstrate a benefit to using the wavefront component in primary LASIK. The authors believe that by use of the POF incorporated into the CRS-Master, a counterbalancing of the relatively high amplitude higher-order spherical aberrations induced by the flap can be achieved, allowing the naturally occurring higher-order aberrations to become less embedded.
Prolate optimization function: MEL80 application
Preliminary investigation into the effect of prolate optimization independent of wavefront customization on the efficacy and safety of myopic LASIK data was reported by Reinstein and Srivannaboon [36]. In a prospective evaluation of LASIK for myopic astigmatism in 60 consecutive eyes, spherical equivalent mean (± SD) myopia treated was −4.51 D (±1.88 D) with a range extending to −9.38 D. Patients were fully evaluated at 1 week, 2 months, and 5 months by an independent observer. Accuracy analysis at 6 months showed a mean postoperative spherical equivalent of −0.05 D, with 90% of eyes within 0.25 D and 100% within 0.5 D of intended correction. The predictability (described by the SD of the mean spherical equivalent) at 5 months was 0.18 D. Efficacy analysis reflected this level of accuracy and predictability, with 94% of eyes seeing 20/20 or better, 97% seeing 20/25 or better, and 100% seeing 20/32 or better. Orbscan II (Bausch & Lomb), using software version 3.00E, was used to derive front surface asphericity (Q factor) from the best fit central 4-mm zone before and after surgery. The mean Q-factor changed from a preoperative mean value of +0.030 to a postoperative mean value of +0.015 with a root-mean-square (RMS) change of +0.075 from before to after surgery. This outcome is in contrast to results of a similar myopic cohort of 53 eyes treated with a conventional myopic ablation profile where Q value changed from −0.009 to +0.089 with an RMS change of +0.531 from before to after surgery. POF served to decrease the increase in Q, or decrease in prolateness of the cornea (DZ Reinstein, personal communication, 2003). This result in turn would be expected to correlate with a reduction in the induction of spherical aberration.
These MEL80 outcomes are significantly better than those achieved by LASIK performed with the MEL70, and it is believed that much of the improvement comes from a better understanding of corneal tissue responses to flap creation and photoablation. Such an optimized non-wavefront-guided system, we believe, forms a substantial platform for the overlay of the high quality wavefront data provided by the WASCA aberrometer.
MEL80 True Wavefront Guided Outcomes: preliminary results
With the establishment of improved optimization in the correction of lower-order aberrations, and closed-loop WASCA-MEL80 induction and removal of aberrations in plastic, the authors set out to study whether the incorporation of higher-order aberrations into the prolate optimized profiles would be of further benefit. Thus they were to test whether True Wavefront Guided treatments were any different from simple prolate optimized treatments. To the authors' knowledge, this specific clinical trial has never been published previously. Seventeen consecutive patients recruited for the study were given a MEL80 standard prolate optimized treatment in the dominant eye while receiving a CRS-Master-derived True Wavefront Guided profile in the nondominant eye, which combined prolate optimized treatment of the lower-order and higher-order aberrations. Preoperatively, the right and left eyes of each patient were similar with respect to mean and SD of the spherical equivalent (P = 0.86). At a minimum follow-up of 3 months, there were no statistically significant differences between eyes in efficacy (UCVA), accuracy, or safety (changes in best spectacle corrected visual acuity). However, there were improvements noted in the higher-order aberration structure of True Wavefront Guided eyes postoperatively. Fig. 15 shows a plot of spherical aberration change for one of the study patients in whom the eye treated with prolate optimization demonstrates an increase in spherical aberration, while the eye treated by True Wavefront Guided ablation shows virtually no increase. There was a highly statistically significant drop in the induction of spherical aberration in the True Wavefront Guided eyes (P = 0.001), with the True Wavefront Guided eyes displaying 32% less induction in spherical aberration (measured at a 6.5-mm pupil size). These results were presented at the Fifth International Congress of Wavefront Sensing and Optimized Refractive Corrections, February 21 22 23, 2004, Whistler, Canada; the full study will be published in due course with more follow-up. Thus it was demonstrated that True Wavefront Guided treatments may indeed offer a benefit over standard prolate optimized treatments. Further study to determine the visual benefits of this difference in terms of contrast sensitivity, modulation transfer function, and point spread function is under way.
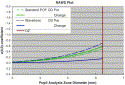
|
|
Fig. 15. Plot demonstrating spherical aberration (Z[4,0]) as a function of pupil analysis zone for the eyes of a patient before and after LASIK using a Prolate Optimized treatment in one eye (green lines) and True Wavefront Guided treatment in the other eye (blue lines) with the WASCA, CRS-Master and MEL80 platform. The dashed lines represent the spherical aberration before LASIK, while the solid lines represent the change in spherical aberration (Z[4,0] postop −Z[4,0] preop). The “standard” MEL80 Prolate Optimized treatment eye encountered a much larger change in spherical aberration than the True Wavefront Guided eye (solid green versus solid blue lines). At the treatment zone diameter of 6.25 mm, the percentage increase in spherical aberration was 105% for the Prolate Optimized treated eye but only 20% for the True Wavefront Guided eye. |
Summary
The Carl Zeiss Meditec platform for custom ablation incorporates a suite of technology for wavefront aberrometry (WASCA), corneal surface shape data (TOSCA), sophisticated excimer laser delivery (MEL80), and surgeon-controlled individualization of treatment protocol (CRS-Master). Together, these components promise to deliver increasingly higher accuracy and control over corneal sculpting—a dream come true for the father of keratomileusis, the late Jose Ignacio Barraquer.