-Courtesy Kevin K Leidl M.S, George H Pettit M.S, PhD
The CustomCornea platform (Alcon, Orlando, Florida) is a wavefront-guided treatment technology. Although laser vision correction with the LADARVision system (Alcon, Orlando, Florida) has been commercially available for several years, the wavefront-based method is relatively novel. In the latter approach, optical imperfections in the eye are diagnosed with a wavefront sensing instrument called the LADARWave, rather than with a conventional Phoropter. The wavefront sensing method can detect subtle visual problems, called high-order aberrations, in addition to classical myopia, hyperopia, and astigmatism [1]; therefore, the wavefront device provides a very detailed picture of the optical functioning of the eye. This detailed information can be used to design a customized ablation profile that will treat lower- and higher-order aberrations.
Assessment of how well CustomCornea technology works in practice raises a two-part question: (1) How well does the LADARWave aberrometer measure visual problems in real eyes? (2) How good a job does the LADARVision system do in treating the measured aberrations?
With regard to the first question, clinical aberrometers face the challenging task of measuring sometimes extremely large aberrations very accurately. To demonstrate the measurement capabilities of the LADARWave device, two case examples are presented herein: (1) a patient with moderate keratoconus and (2) another with prior eight-incision radial keratotomy surgery. These eyes were chosen in particular because they have substantial amounts of very different higher-order aberrations.
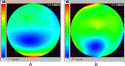
In the keratoconic eye, the bulge in the inferior cornea increases the local curvature and hence the refractive power in that region. The eye is more myopic inferiorly than superiorly, producing substantial vertical coma. (Coma can roughly be described as one half of the pupil being more myopic and the other half more hyperopic than the overall average.) Fig. 1 shows a LADARWave wavefront analysis for an eye with moderate keratoconus. Note in the power map (Fig. 1B) that the maximum myopic power exceeds −17 D and is rapidly changing over the lower pupil. This patient example demonstrates that the instrument can measure marked aberrations without difficulty.
 |
|
Fig. 1. LADARWave system wavefront analysis for an eye with moderate keratoconus. (A) Two-dimensional pseudocolor display of the wavefront profile. The eye is below the plane of the figure. The color scale is shown on the left side of the panel, with the units in microns. Red indicates the highest points in the profile, whereas blue shows the deepest depressions. (B) Instantaneous power map calculated directly from the wavefront axial curvature. The colors indicate instantaneous refractive power in diopters, with red indicating the most hyperopic and blue the most myopic regions. Both maps cover the same 7-mm diameter circle at the cornea and are based on an eighth-order Zernike polynomial reconstruction. |
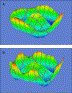
The second example is from an eye that has undergone prior radial keratotomy with an eight-incision surgery. Fig. 2 shows two three-dimensional wavefront reconstructions for this case. Notice that both profiles have a general “sombrero” shape owing to spherical aberration, which is the most significant single aberration for this eye. Note also the obvious differences between the wavefront descriptions (Fig. 2A,B). The eighth-order reconstruction (Fig. 2B) looks almost like a flower owing to the nature of the surgical procedure where eight relaxing incisions lie at the edge of this measurement region. The cuts cause general but nonuniform flattening of the central cornea and produce a slight periodic ripple in the surface. Viewing topographic data just along the circumference of this measurement circle would reveal eight local peaks and valleys. The fluctuations produce refractive undulations that are described by two eighth-order Zernike terms. The LADARWave device can provide a fine level of detail by allowing analysis to up to eight Zernike orders.
 |
|
Fig. 2. Three-dimensional wavefront displays for an eye with prior eight-incision radial keratotomy surgery. (A) Wavefront reconstruction using up to fourth-order Zernike polynomials. (B) Wavefront reconstruction using up to eighth-order Zernike terms. The wavefront diameter in both panels is 6.5 mm. |
The second question regarding assessment of the CustomCornea platform can be answered by looking at data from US Food and Drug Administration (FDA) clinical trials of wavefront-guided treatments. The first of these surgeries was performed in the fall of 1999 and, to date, more than 1000 eyes have been treated under various protocols. These cases included myopic and hyperopic eyes treated with either laser in situ keratomileusis (LASIK) or photorefractive keratectomy. In the fall of 2002, the study was expanded to include therapeutic treatments, that is, wavefront-guided surgery for patients with pronounced visual symptoms owing to large higher-order aberrations. At the end of October 2002, the authors received the first FDA wavefront treatment approval for the indication of spherical myopia (ie, myopia with less than 0.5 D of astigmatism).
The initial approval cohort consisted of 139 eyes with a mean preoperative spherical equivalent refraction of −3.23 ± 1.31 D. All of the patients received a 6.5-mm diameter optical zone with a 1.25-mm wide blend zone for a total 9-mm circular ablation region on the eye. Six months after surgery, 58% of the eyes had a mesopic uncorrected visual acuity (UCVA) of 20/16 or better, and 80% had a mesopic UCVA of 20/20 or better. Two points are important to stress here. First, these percentages are based on 100% follow-up of all eyes enrolled after a single treatment. No retreatments were allowed during the follow-up interval. Second, unlike in commercial cases, no nomogram adjustments were allowed in these surgeries. All of the patients were treated in exactly the same way at all centers.
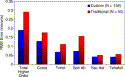
A matched group of 50 traditional LADARVision eyes was treated under the exact same conditions to assess the difference in outcomes as compared with wavefront-based treatment. Fig. 3 shows a comparison of the higher-order aberrations for the two groups at 6 months. This graph clearly shows less higher-order aberrations remaining for the custom treatments. Table 1 shows how the individual higher-order aberrations changed from the preoperative findings for the two groups, with the percent and micron change again highly favorable toward the custom group.
 |
|
Fig. 3. Comparison of higher-order aberrations 6 months after either wavefront-guided or traditional LASIK surgery over 5 mm. Significantly less higher-order aberrations remain with the custom group for each of the coefficients through the fourth order, with the exception of secondary astigmatism (P = 0.098). |
|
Table 1.
Change in aberrations from preoperative level |
|
|
Aberration (5 mm) | Six month mean change |
|
|||
|---|---|---|---|---|---|---|
|
|
Wavefront-guided (n = 139) | Traditional (n = 50) |
|
|||
|
|
μm | % | μm | % |
|
|
|
|
Higher-order | 0.03 | 18 | 0.13 | 77 |
|
|
|
Coma | 0.02 | 22 | 0.08 | 78 |
|
|
|
Trifoil | −0.01 | −11 | 0.03 | 38 |
|
|
|
Spherical aberration | 0.01 | 22 | 0.08 | 108 |
|
|
|
Secondary astigmatism | 0.02 | 74 | 0.03 | 105 |
|
|
|
Tetrafoil | 0.02 | 82 | 0.03 | 119 |
|
The results shown in Table 1 are average values for the different groups of eyes. On an individual patient basis, the total higher-order wavefront error actually decreased in 38% of the wavefront-guided treatments compared with 19% of the traditionally treated eyes. Spherical aberration was reduced in 46% of the custom surgeries compared with 12% of the conventional group.
The difference in the amount of total higher-order aberrations remaining in the two groups 6 months after surgery equates to about 0.2 D of defocus. Fig. 4 shows the simulated impact on a visual acuity chart for the average residual higher-order aberrations for custom treatment on the left and traditional surgery on the right. Postoperative refractive analysis revealed an average residual myopia of −0.4 D across the initial 139-eye cohort. This myopia was compensated for in a subsequent study of 141 eyes. For this refined treatment group, the preoperative sphere was −2.65 ± 1.18 D. Cylinder was also incorporated in this group, with an average of −0.77 ± 0.63 D (range, 0 to −2.50 D), and the spherical equivalent was −3.03 ± 1.18 D.
|
Fig. 4. Optical simulation showing the difference in higher-order aberrations present in the eye after CustomCornea (A) and traditional LADARVision LASIK surgery (B). The difference in the amount of total higher-order aberrations remaining between the two groups 6 months after surgery equates to about 0.2 D of defocus. |
For the refined group at 6 months, the mean manifest refraction spherical equivalent (MRSE) was −0.03 D, with 94% of patients within 0.5 D of emmetropia and 99% within 1.0 D. In comparison, in the initial spherical treatment group, 69% of all eyes were within 0.50 D at 6 months, and 94% were within 1.0 D, with the mean MRSE of −0.4 D. Removal of the undercorrection also resulted in improved UCVA outcomes, with 69% of eyes showing 20/16 or better and 91% showing 20/20 or better for the refined group.
Both populations did well based on other objective clinical measures. Best-corrected visual acuity (BCVA) at 6 months for the initial group was 20/12.5 in 32%, 20/16 in 92%, and 20/20 in 100%. In comparison, preoperative values were 18%, 86%, and 99%, respectively. Four times as many eyes had a gain of greater than or equal to one line of vision than had an equivalent loss. For the refined group, the shift toward improved BCVA was even more dramatic, with a doubling of the number of eyes that were 20/12.5 and a 21% increase at 20/16. Importantly, there was no loss of 20/20 after surgery.
Contrast sensitivity testing was performed before and after surgery. A clinically significant change in contrast sensitivity was defined as greater than 0.3 log units (ie, a difference from preoperative to postoperative of at least two test intervals) at two or more spatial frequencies. By this criterion, in the initial group under photopic conditions, 2% of the eyes had a clinically significant loss from the preoperative state, and 2% had a clinically significant gain in contrast. Under mesopic conditions, 5% of the eyes in the initial treatment group lost contrast sensitivity and 15% gained. Contrast sensitivity testing in the refined treatment group indicated that 2% lost but 5% gained contrast under photopic conditions, and 8% lost but 18% gained under mesopic conditions. In general, photopic contrast sensitivity was virtually unchanged, but mesopic performance generally improved after treatment.
Alcon technology
Fig. 5 shows the Alcon devices that make up the CustomCornea technology. The LADARWave device uses a Shack-Hartmann wavefront sensor to detect the preoperative aberrations present in the eye [1]. The LADARVision system employs an active closed-loop, eye-tracking module to compensate for eye motion and a small-diameter flying spot excimer laser to deliver the customized ablation profile [2]. These two devices work together in a coordinated fashion to provide an effective customized treatment. This technology is based on four guiding principles:
 |
|
Fig. 5. The Alcon CustomCornea technology components. The LADARWave aberrometer, shown in the upper left, measures the optical characteristics of the eye using the Shack-Hartmann wavefront sensing approach. The LADARVision system treats the measured aberrations using an active eye-tracking system and a small flying spot excimer laser. (Courtesy of LADARWave, Alcon Lab, Fort Worth, Texas.) |
Reference axis
The first guiding principle is that a consistent reference axis should be used throughout the customized treatment process. Fig. 6 shows simplified views of the fixation and video alignment subsystems in the LADARWave aberrometer and LADARVision system. For either instrument, as the patient stares at an internal fixation target, a video camera looks at the eye along the same optical axis. This common design greatly facilitates the definition and use of a consistent axis on the eye.
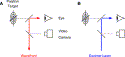 |
|
Fig. 6. Fixation and video schemes for the LADARWave (A) and LADARVision (B) systems. The commonality of these two designs allows a consistent geometric reference axis to be defined on the eye and used throughout the measurement and treatment steps. |
The reference axis used is the natural line of sight. The line of sight is defined as the line connecting the point of fixation and the center of the pupil [3]. The natural (undilated) rather than dilated pupil is used as the reference because dilation, particularly pharmacologic dilation, can cause a significant shift in the pupil center [4].
The LADARWave unit records the natural line of sight as a preliminary step in the wavefront examination, although any point of centration can potentially be specified. The instrument captures a video image of the eye as the patient looks at an internal fixation target under daytime illumination conditions. This video snapshot is presented on screen to the device operator, and he or she aligns two software reticles over the pupil margin and limbus. From this time onward, the device software knows where the natural pupil center sits on the eye relative to the limbus. After the patient's eye is dilated, the eye can still be centered along this axis for the actual wavefront measurement. This centration information is also passed on to the LADARVision system in the electronic transfer file so that the ablative treatment can be centered over the natural pupil as well.
Aberration data
The second guiding principle is that accurate aberrometry must be performed over a region that encompasses the naturally dilated pupil. This task requires good fixation on the part of the patient, dilation of the eye with pharmacologic agents, and high-fidelity wavefront measurement by the LADARWave device.
Fixation
The patient must know exactly where to look during the wavefront examination to provide the best possible data. Although the aberrations of the eye are relatively constant over small field angles [5], the most relevant wavefront data are obtained when the probe beam illuminates a central foveal region. Most patients do not have good unaided visual acuity before surgery; therefore, they have difficulty seeing a static target at infinity focus. Some patients may find the target by accommodating, but this will substantially alter the wavefront profile [6]. The fixation path must have adjustable compensating optics so that each patient can visualize the target with the eye in a relaxed state.
The LADARWave device clarifies the target automatically for each eye by adjusting spherocylindrical lenses to correct for conventional refractive errors. The software accomplishes this task by analyzing preliminary wavefront data in real time at the beginning of the examination. The instrument can compensate for spherical errors ranging from +15 to −15 D and astigmatic errors up to −6 D. (Patients with somewhat larger refractive errors can still be measured, but the target clarity will not be optimal.) Once the target is clear, the software “fogs” it slightly, that is, it moves it in the hyperopic direction to minimize accommodation. The cylinder compensation is particularly important in ensuring that the correct fogging is provided to astigmatic patients. This automatic process works well and lessens the workload on the device operator considerably. The automatic fogging routine is also used during the centration step discussed in the previous section.
Dilation
The pupil is the limiting aperture for the wavefront measurement, and aberration data cannot be obtained beyond the pupil margin. In fact, valid data cannot even be obtained over the entire pupil extent because the iris aperture partially distorts the peripheral measurements. Obtaining peripheral data is an important consideration given that higher-order aberrations typically become more pronounced near the pupil periphery.
Pharmacologically dilating the eye expands the region of valid wavefront data to encompass the largest natural pupil completely. Alcon has conducted careful clinical testing to study the effects of drug dilation on the aberration profile [7]. The only notable pharmacologic effect on the wavefront is a small hyperopic shift in the defocus term. This defocus shift is more common with hyperopic patients. The higher-order aberrations are unaffected by the dilating agent. This consistency has been observed in every eye tested, showing the validity of measuring the wavefront under drug dilation. Studies using other wavefront sensors have also measured the effects of cycloplegia on wavefront aberrations, with some finding differences in higher-order aberrations [8]. One of the key features of the LADARWave aberrometer is that it provides the means to rebuild the wavefront about a defined position on the eye regardless of cycloplegia. This ability negates the potential effects of pupil center shift with dilation, which otherwise could significantly influence higher-order aberrations.
Wavefront measurement
The LADARWave device uses the Shack-Hartmann approach to measure the aberrations in the eye. This method is described in detail elsewhere in this issue. The Shack-Hartmann measurement produces a camera image showing a pattern of dots, which are the individual wavefront pieces divided and focused by the lenslet array. The device software measures the displacement of each focused dot from its ideal location, that is, the pattern generated by a perfectly flat plane wave, and uses this information to calculate the slope of the intact wavefront at each lenslet location. The software then uses this slope data to generate a mathematical description of the original wavefront profile.
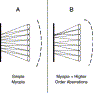
Although this technology is simple in concept, much care must be taken in building a clinical aberrometer that is reliable, easy to operate, and highly accurate in measuring the wide variety of visual aberrations found among refractive surgery patients. Fig. 7 shows the detection of two significantly myopic wavefronts by a Shack-Hartmann sensor. In a good clinical device, the software must be able to analyze large spot displacements in the CCD camera image (Fig. 7A, B). In addition, the optical hardware design must minimize the chance of spot crossover (Fig. 7B).
 |
|
Fig. 7. Shack-Hartmann detection of two different myopic wavefronts. The wavefronts are propagating from right to left. (A) Simple myopic wavefront with no higher-order aberrations. The spacing between individual focused wavefront elements on the CCD is uniform but smaller than the lenslet array spacing. (B) Complex wavefront including higher-order aberrations. The focused dot pattern here is no longer uniform. In addition, the two wavefront elements at the top have crossed over each other in traveling from the lens array to the screen. This measurement is compromised in this region. |
After much clinical testing and engineering innovation, Alcon has been able to address such issues and has produced an instrument that provides high accuracy and a large dynamic range, along with high-density sampling of the wavefront across the pupil. Although some details are proprietary, the performance characteristics are not. The LADARWave unit uses a square lenslet array geometry and samples the eye approximately every 420 μm at the corneal plane. For a 7-mm diameter pupil, this results in more than 200 wavefront samples at the Shack-Hartmann sensor, which equates to over 400 data values, because each sample provides wavefront slope information in two directions. This large body of data permits very accurate calculation of wavefront aberrations up to the eighth order, which encompasses 44 terms. The device can measure wavefronts with a maximum curvature in any meridian lying between +8 and −14 D (defined for an 8-mm pupil). The unit can also measure up to 8 D of astigmatism. The sphere and cylinder accuracy over this measurement range is 1% of the actual value or 0.05 D (whichever is larger). On top of the classical refractive error, the device can measure large amounts of higher-order aberrations.
A typical wavefront examination consists of five individual measurements. The LADARWave software compares the five wavefront profiles automatically, and the three in closest agreement are then averaged together to generate a composite final wavefront. This process eliminates transient fluctuations in the aberration content owing to microsaccadic eye movements or dynamic action of the lens.
The LADARWave device uses the synchronized video imagery to help the operator determine the validity of the wavefront measurement. Fig. 8 shows a video image frozen at the instant a wavefront measurement was made. In this example, the patient has begun to blink just as the probe beam fires. Maintaining an intact tear film is essential in the wavefront examination [9]; therefore, the instrument must accommodate normal blinking by the patient. The upper lid will occasionally get in the way. By automatically projecting the wavefront data region onto the picture, the device software provides a clear indication that this measurement is not representative of the full pupil and should be rejected. Intracorneal or intraocular opacities (eg, scars, cataracts, vitreous strands) may also obscure part of the re-emitted wavefront, and such conditions will also be revealed in this synchronized video display.
 |
|
Fig. 8. Frozen video image taken simultaneously with a wavefront measurement. The crosshatched region in the picture is the area providing wavefront data, as determined by the device software. Because the pupil is the limiting aperture for a good measurement, the crosshatched area should closely approximate that of the pupil. In this instance, the top of the pupil is partially occluded by eyelashes. |
Registration
The third guiding principle is that the wavefront data must be registered accurately to the anatomy of the eye so that the ablative correction can be applied to the correct corneal location. On the wavefront measurement side, this registration is accomplished using synchronized video imagery. The video and Shack-Hartmann cameras look at the pupil of the eye along the same optical axis; therefore, there is a direct one-to-one correspondence between each point in the video and wavefront camera images. At the instant that a wavefront measurement is taken, the LADARWave software freezes the video picture of the eye. The device operator aligns two reticles (limbus and linear) in the frozen video image, as shown in Fig. 9. This maneuver tells the software the exact position and cyclotorsional state of the eye at the instant the wavefront was captured. The process is repeated for each of the five measurements in the complete examination, and the reticle data are then used in calculating the composite wavefront profile. The reticle information is also transmitted in the electronic file over to the LADARVision system so that the ablative treatment can be aligned properly.
 |
|
Fig. 9. Method of registering the wavefront geometry with the anatomy of the eye. Just before the wavefront examination, multiple ink marks are applied to the sclera. In the frozen video image of the eye (taken simultaneously with the wavefront measurement), software reticles are aligned to these marks and the limbus. This registration provides the system with accurate position and cyclotorsion information. |
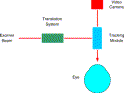
On the treatment side, the registration process takes advantage of the LADARVision system's closed-loop, eye-tracking device. The optical block diagram of the treatment device is shown in Fig. 10. The video camera looking through the tracking mirrors sees the eye after any motion has been compensated for. The LADARVision graphical user interface presents this “space stabilized” video image to the system operator along with registration reticles identical to those used during measurement by the LADARWave system. The operator repositions the on-screen reticles over the appropriate anatomic landmarks to ensure accurate delivery of the excimer ablation profile.
 |
|
Fig. 10. Optical strategy employed in the LADARVision system. The tracking module contains a dedicated set of mirrors, tasked solely with compensating for detected eye motion. The video and excimer subsystems are optically coupled into the tracking module so that they interact with a space-stabilized eye. The translation system contains a second set of mirrors, whose only job is to deliver the excimer beam in a predetermined pattern of pulses based on the patient's aberration data. |
Cyclotorsion is a key but often overlooked factor in complete wavefront registration. With conventional surgery, it is well known that torsional alignment of the ablation is important in correcting cardinal (“second-order”) astigmatism. A torsion error of 10 degrees results in a residual astigmatism that is approximately one third of the initial astigmatism magnitude [10]. With higher-order treatments, the required torsional accuracy becomes even more critical, dependent on the azimuthal frequency of the wavefront aberration.
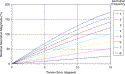
The previous statement merits a brief explanation. Zernike polynomials increase in complexity with increasing order. Only a small fraction of these polynomials are rotationally symmetric (ie, have no angular dependency). Examples of rotationally symmetric Zernike shapes include defocus and spherical aberration. Nonrotationally symmetric terms, such as coma or tetrafoil, have an undulating height around their perimeter. The azimuthal frequency is directly related to the number of these up or down undulations. In an attempt to treat one of the rotationally symmetric aberrations, the presence of a torsion error will have no effect, because the shape stays the same upon rotation. In contrast, a torsional error will have a substantial effect on the treatment of nonrotationally symmetric aberrations, as is shown in Fig. 11.
 |
|
Fig. 11. Effect of torsional error on the correction of nonrotationally symmetric aberrations. The horizontal axis indicates the rotational error in delivering an ablation profile to the eye. The vertical axis indicates the percentage of a particular aberration that will be left after the surgery. The different colored plots indicate the relationship for aberrations with different azimuthal frequencies according to the legend at the right of the plot. Note that the vertical axis goes beyond 100%, indicating that some aberrations may actually be increased by a poorly registered customized ablation. |
Cyclotorsion takes on additional significance considering that the aberration examination is performed with the patient sitting upright. He or she must then lie down for surgery, and the apparent rotational state of the eye may change significantly. Unless this rotation is accounted for, it will degrade the wavefront-based correction. The LADARWave/LADARVision system technology allows the operator to register the wavefront very accurately in position and angle relative to the anatomy of the eye.
Conversion
The fourth guiding principle behind the CustomCornea platform concerns the algorithm that converts the aberration data into the ablation profile. This algorithm should be optimized to provide best optical performance over the optical zone and graceful tapering of the ablation in the surrounding blend zone.
The treatment algorithm resides in the LADARVision system. Because it is proprietary, a detailed explanation cannot be provided herein. A “first-order” approximation is shown in Fig. 12. The optical path difference approach shown here represents a good starting point in determining the correction needed; however, it is not the complete method used in CustomCornea treatments. Additional factors such as corneal curvature [11] and corneal biomechanics [12] must be taken into account in optimizing the ablative treatment.
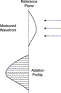 |
|
Fig. 12. “First-order” approach in determining the appropriate ablation profile from the wavefront shape. The upper part of the figure shows a generally myopic wavefront (traveling to the left) compared with the idealized (flat) reference plane. The difference between the real wavefront and an ideal plane is calculated at each point in the examination plane. This optical path difference (OPD) profile is then multiplied by −1 and divided by the refractive index difference between cornea and air. Because light travels slower in corneal tissue than in air, ablating the most cornea centrally will “speed up” the central wavefront with respect to the edges, producing a flatter aberration profile. |
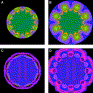
Fig. 13 shows the importance of surrounding the optical zone with a customized blend annulus. Both ablation profiles are attempting to treat the Z88 “flower” aberration over a 6.5-mm diameter optical zone. This aberration is relatively flat centrally, with eight undulating ripples evenly spaced around the perimeter. Without a blend zone, the resulting corneal surface (panel C) has finite height errors within the optical zone and significant irregularities just outside it. Adding the blend zone produces a more regular corneal surface in the periphery; it also improves the peripheral treatment accuracy within the optical zone. Because “flying spot” excimer ablation relies on the cumulative effect of many partially overlapping pulses, shots delivered just outside the optical zone contribute to the correction just inside it. The wavefront root mean square (RMS) error corresponding to the ablation outcome in panel C is 70% of the pretreatment value. In contrast, if a blend zone is included in the ablation profile, the RMS wavefront error is reduced to 36% of the preoperative level.
 |
|
Fig. 13. Effect of a customized blend zone on the treatment accuracy. (A) Color map indicating the ablation profile needed to correct a particular eighth-order aberration over a 6.5-mm circular optical zone with no blend. (B) Map showing the same treatment with a 1.25-mm blend zone annulus added around the optical zone. (C) Simulated corneal treatment of the ablation profile shown in panel (A), using a small-diameter (750-μm FWHM) excimer laser. Accurate correction is indicated by the uniform bluish color near the center of the image. Residual corneal height errors are indicated by the other colors. (D) Simulated corneal treatment of the ablation profile shown in panel (B). Inclusion of the customized blend zone significantly improves the treatment accuracy. |
The ability of the CustomCornea platform to measure the wavefront accurately and treat the appropriate location on the eye has led to impressive clinical results. The data show excellent visual acuity and more gain and less loss of mesopic contrast sensitivity. The higher-order aberrations for customized eyes are increased in magnitude but remain modest and are significantly less than those seen for conventionally treated eyes. In a comparison with conventional surgery, a much larger percentage of customized eyes show a reduction in higher-order aberrations. The complexity of wavefront-guided corrections places increased requirements on the measurement and delivery technology, and the CustomCornea platform has been developed to meet those requirements.